Not an actual patient.
Streamlined Delivery System
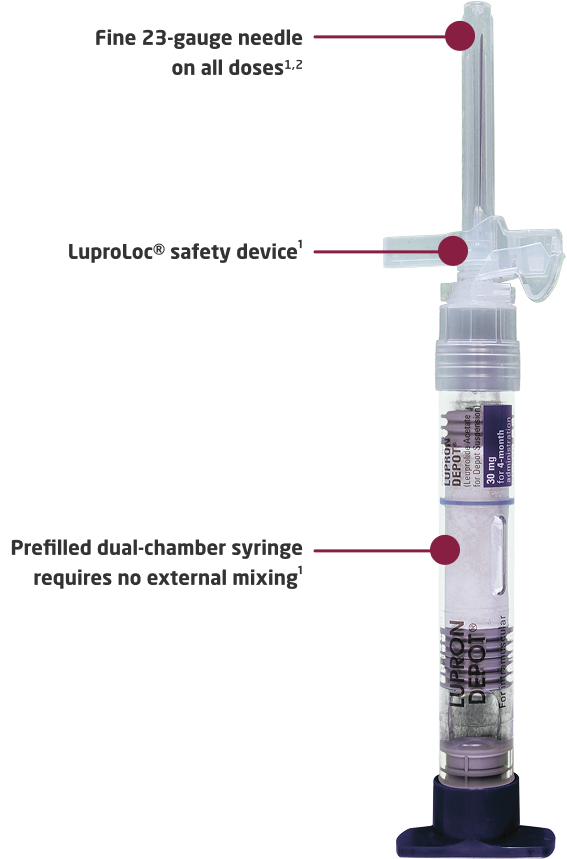
Closed-system transfer device1,3*
*Mechanically prohibits the transfer of environmental contaminants into the system and the escape of hazardous drug or vapor concentrations outside the system.3
Review Product Features
Features of available injectable androgen deprivation therapies.
Information current as of February 2025.
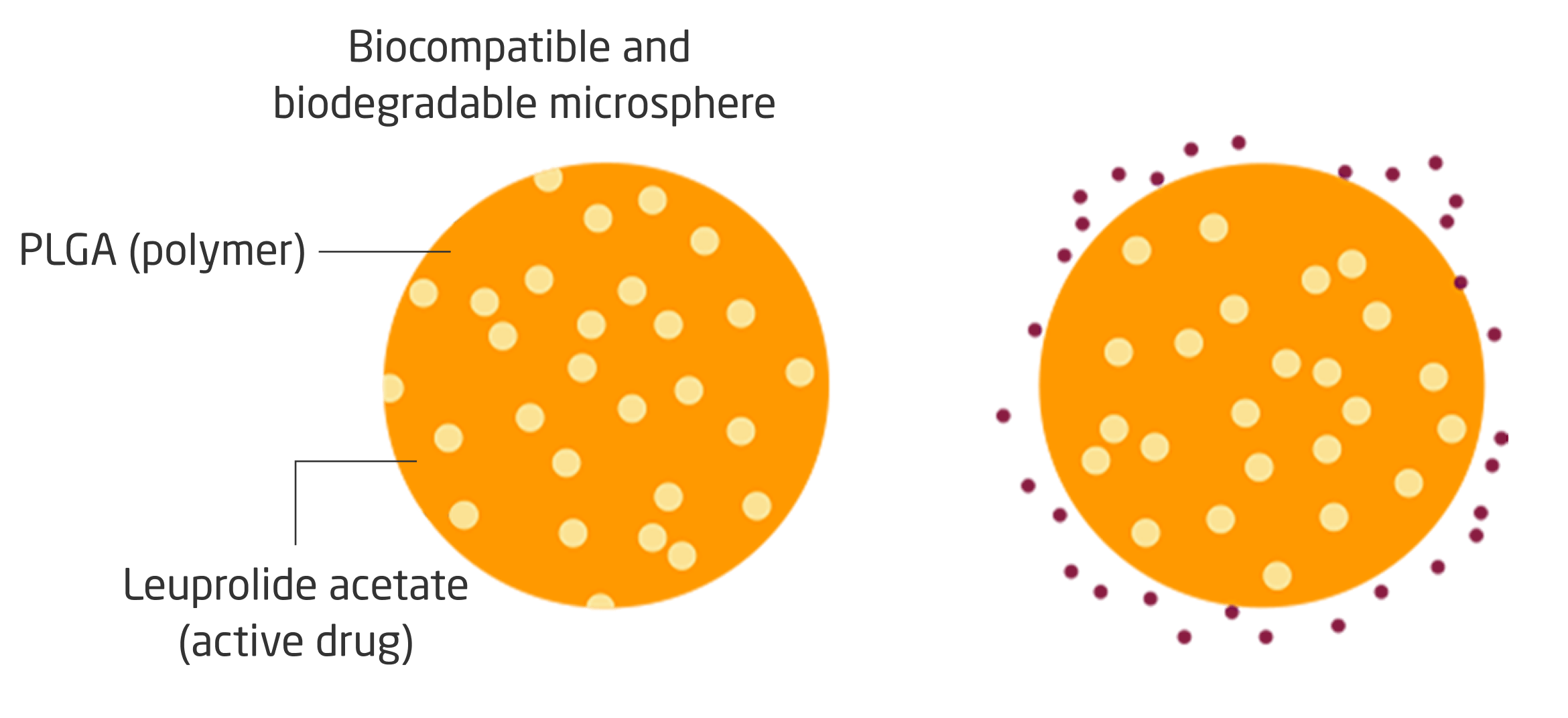
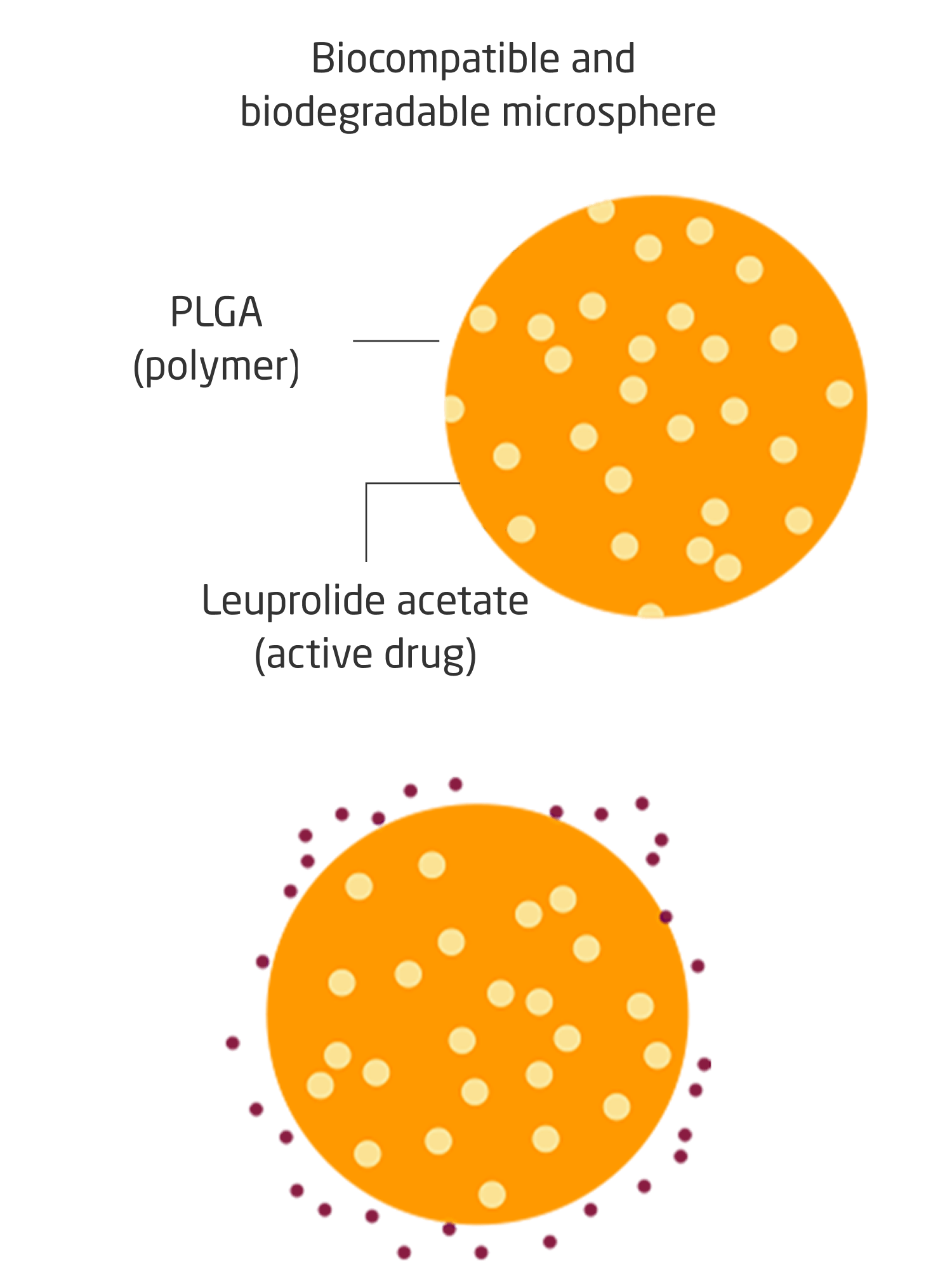
Lyophilized Microsphere Technology
LUPRON DEPOT is formulated with lyophilized microsphere technology, gradually releasing active drug from each microsphere over time.1,11

Diffusion


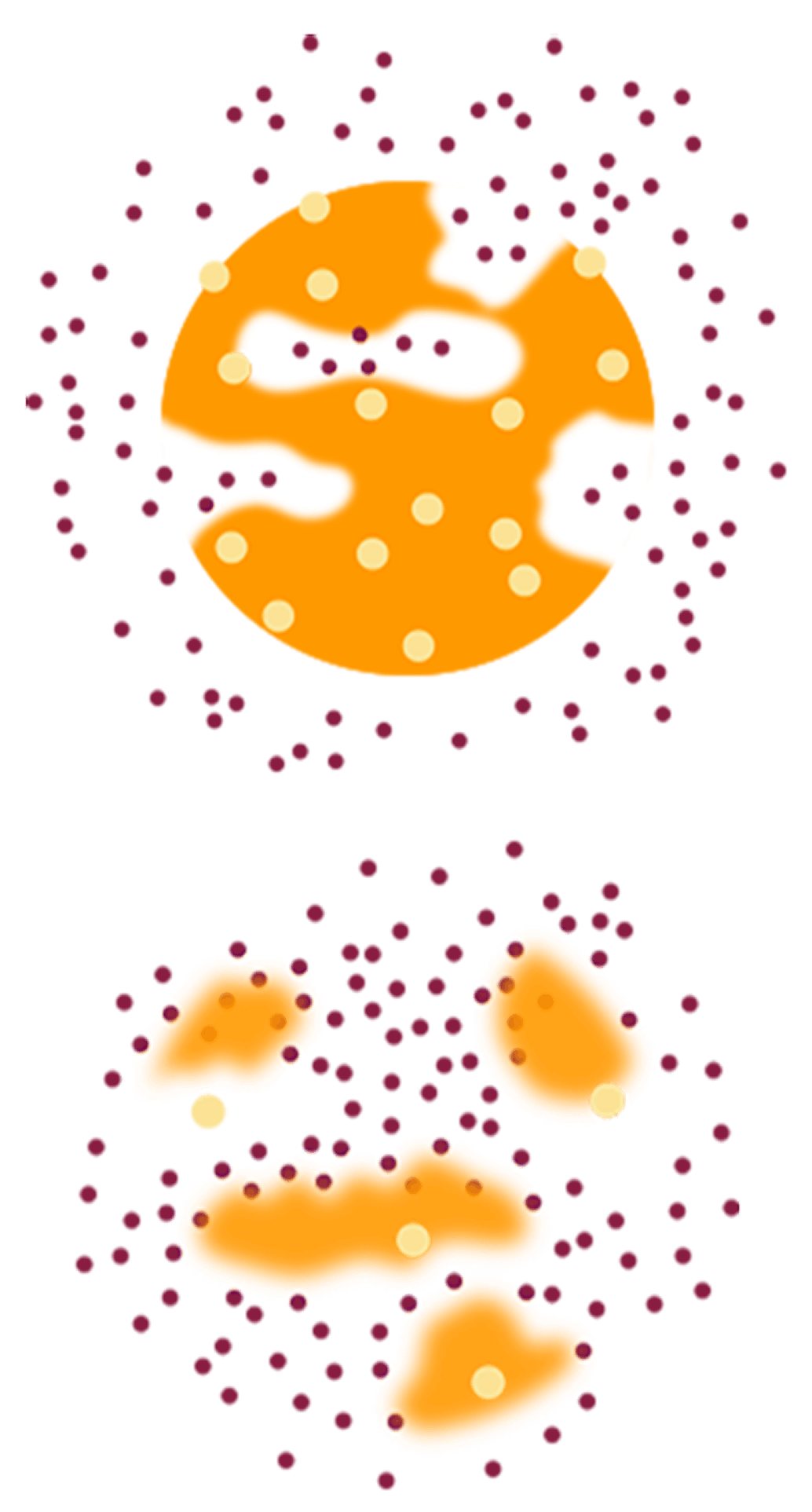
Polymer Bioerosion
- The active drug, leuprolide acetate, is embedded in microcapsules composed of a biodegradable, hydrophobic polymer to overcome high solubility in body fluids and rapid excretion in vivo1,11
- Leuprolide acetate is released in 2 phases in vivo: diffusion and bioerosion, gradually releasing active drug from each microsphere over time1,11
Lyophilized microsphere technology allows for controlled release of leuprolide acetate, designed to suppress testosterone levels1,11
PLGA=poly(lactic-co-glycolic acid).
LuproLink® Inventory Management
Discover LuproLink®—an intuitive inventory management system for LUPRON DEPOT prescribers that delivers important LUPRON DEPOT inventory data and seamless EHR integration.
EHR=electronic health record.

“The innovative delivery system that LUPRON DEPOT provides is appreciated, especially by our staff. Its design keeps both my team’s and the patient’s experience in mind.”



