Not an actual patient.
ON THIS PAGE
LUPRON DEPOT delivered testosterone suppression below castrate levels across multiple formulations, as shown in multicenter clinical trials enrolling patients with histologically confirmed advanced prostate cancer.1
Effective Testosterone Suppression
In an open-label, noncomparative, multicenter study
Testosterone Suppression With 6-Month Dosing1,2
LUPRON DEPOT 45 MG: MEAN SERUM TESTOSTERONE CONCENTRATION THROUGH WEEK 481-3
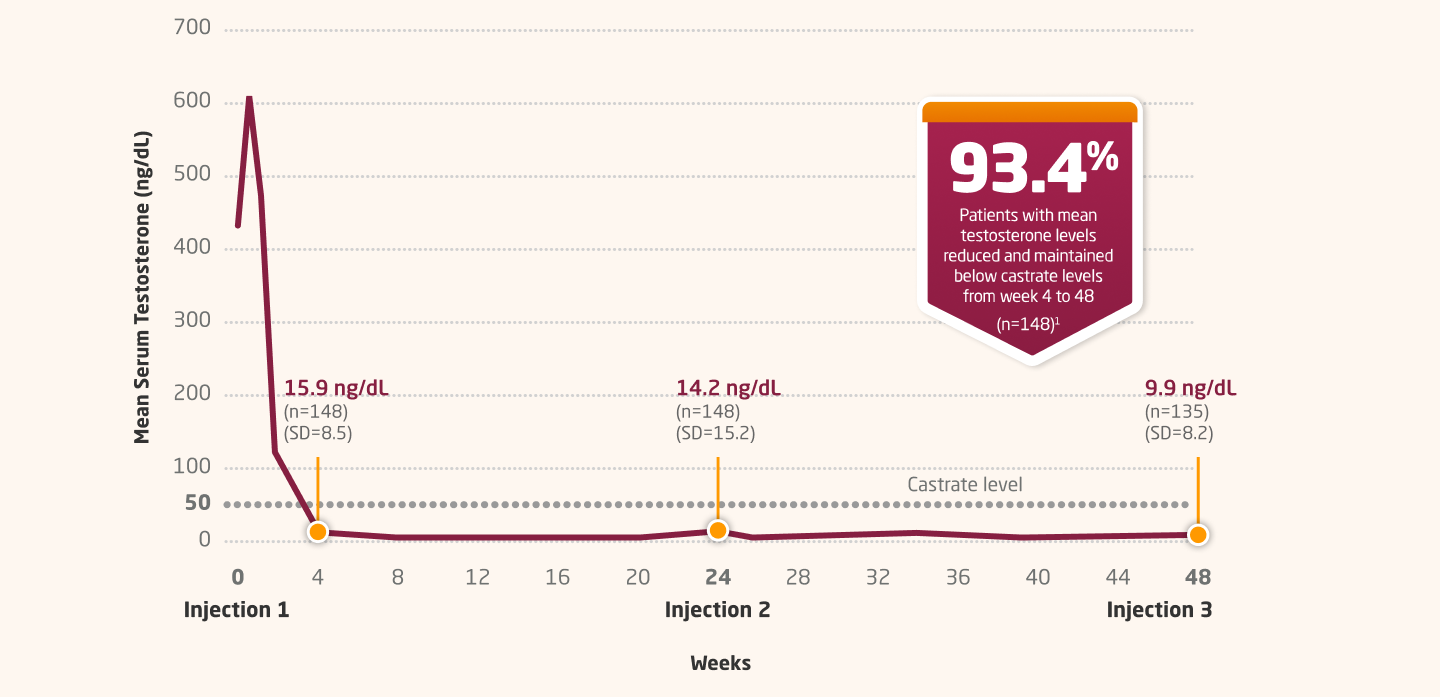
CI=confidence interval; SD=standard deviation.
Primary Endpoint Results1,2
Serum testosterone was suppressed to ≤50 mg/dL from week 4 through week 48 in 93.4% of patients (two-sided 95% Cl: 89.2%, 97.6%; N=148).
Safety Data for 6-Month Dosing
| Adverse Events in ≥ 5% of Patients1 | Treatment Emergent n=151 (%) |
Treatment Related n=151 (%) |
|---|---|---|
| Hot Flush/Flushing | 89 (58.9) | 88 (58.3) |
| Injection Site Pain/Discomfort | 29 (19.2) | 16 (10.6) |
| Upper Respiratory Tract Infection/Influenza-like Illness* |
32 (21.2) | 0 (0) |
| Fatigue/Lethargy | 20 (13.2) | 18 (11.9) |
| Constipation | 15 (9.9) | 5 (3.3) |
| Arthralgia | 14 (9.3) | 2 (1.3) |
| Insomnia/Sleep Disorder | 13 (8.6) | 5 (3.3) |
| Headache/Sinus Headache | 12 (7.9) | 3 (2.0) |
| Musculoskeletal Pain/Myalgia | 12 (7.9) | 3 (2.0) |
| Second Primary Neoplasm † | 11 (7.3) | 0 (0) |
| Cough | 10 (6.6) | 2 (1.3) |
| Hematuria/Hemorrhagic Cystitis | 10 (6.6) | 0 (0) |
| Hypertension/BP Increased | 10 (6.6) | 3 (2.0) |
| Rash | 9 (6.0) | 3 (2.0) |
| Dysuria | 9 (6.0) | 1 (0.7) |
| Urinary Tract Infection/Cystitis | 9 (6.0) | 0 (0) |
| Anemia/Hemoglobin Decreased | 10 (6.6) | 2 (1.3) |
| Back Pain | 8 (5.3) | 0 (0) |
| COPD | 8 (5.3) | 0 (0) |
| Dizziness | 8 (5.3) | 3 (2.0) |
| Dyspnea/Dyspnea on Exertion | 8 (5.3) | 2 (1.3) |
| Nocturia | 8 (5.3) | 2 (1.3) |
| Peripheral/Pitting Edema | 8 (5.3) | 2 (1.3) |
| Coronary Artery Disease/Angina | 8 (5.3) | 1 (0.7) |
*Includes influenza, nasal congestion, nasopharyngitis, rhinorrhea, upper respiratory tract infection, and viral upper respiratory tract infection.
†Includes basal cell carcinoma, bladder transitional cell carcinoma, lung neoplasm, malignant melanoma, non-Hodgkin lymphoma, and squamous cell carcinoma.
BP=blood pressure; COPD=chronic obstructive pulmonary disease.
In an open-label, noncomparative, multicenter study
Testosterone Suppression With 4-Month Dosing
LUPRON DEPOT 30 MG: MEAN SERUM TESTOSTERONE CONCENTRATION THROUGH WEEK 321,4,5
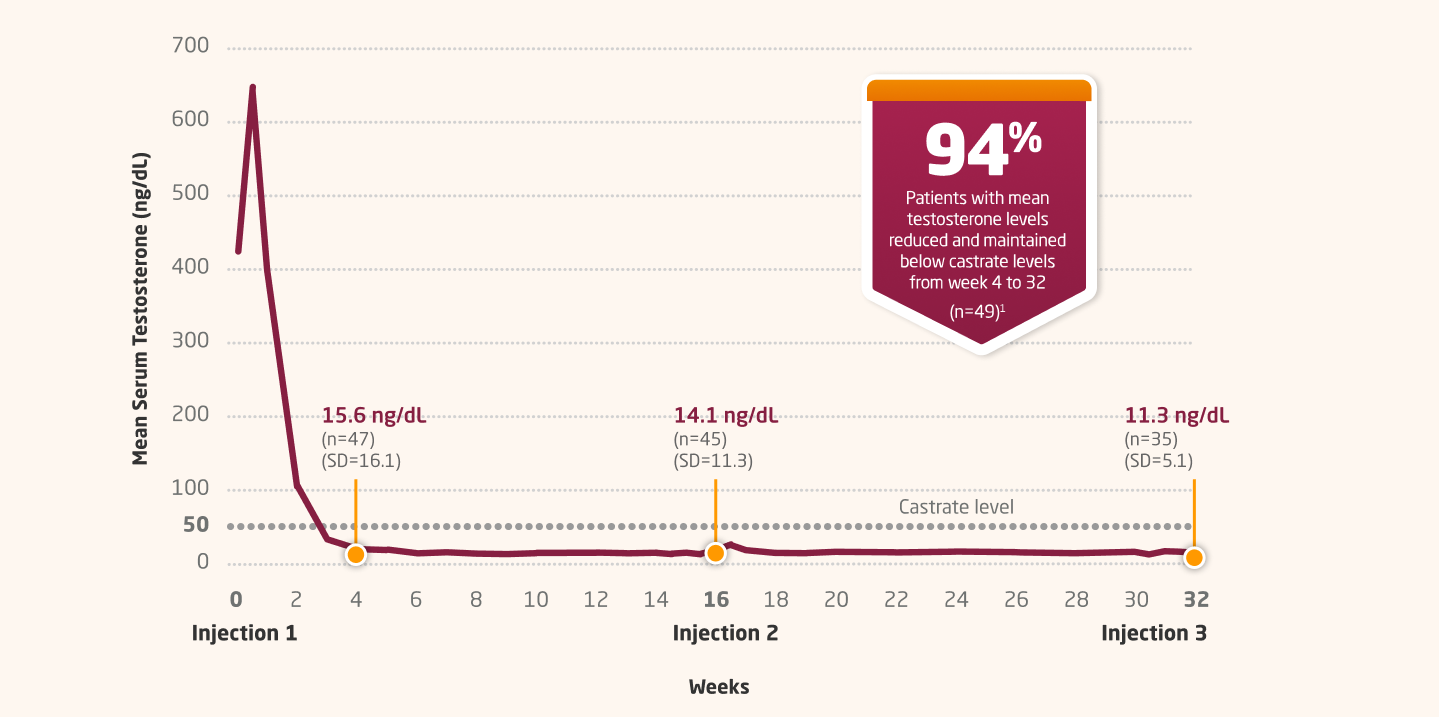
Primary Endpoint Results1,4
Castrate levels were achieved in 94% of patients by day 30 and in all patients by day 43. After falling to castrate range, mean testosterone levels remained within the castrate range throughout each dosing interval.
Safety Data for 4-Month Dosing
The 4-month formulation of LUPRON DEPOT 30 mg was utilized in clinical trials that studied the drug in 49 nonorchiectomized prostate cancer patients for 32 weeks or longer and in 24 orchiectomized prostate cancer patients for 20 weeks.1
| Adverse Events in ≥ 5% of Patients1 | Nonorchiectomized n=49 (%) |
Orchiectomized n=24 (%) |
|---|---|---|
| Body as a Whole | ||
Asthenia |
6 (12.2) |
1 (4.2) |
Flu Syndrome |
6 (12.2) |
0 (0.0) |
General Pain |
16 (32.7) |
1 (4.2) |
Headache |
5 (10.2) |
1 (4.2) |
Injection Site Reaction |
4 (8.2) |
9 (37.5) |
Cardiovascular System |
||
Hot Flashes/Sweats |
23 (46.9) |
2 (8.3) |
Digestive System |
||
GI Disorders |
5 (10.2) |
3 (12.5) |
Metabolic and Nutritional Disorders |
||
Dehydration |
4 (8.2) |
0 (0) |
Edema |
4 (8.2) |
5 (20.8) |
Musculoskeletal System |
||
Joint Disorder |
8 (16.3) |
1 (4.2) |
Myalgia |
4 (8.2) |
0 (0) |
Nervous System |
||
Dizziness/Vertigo |
3 (6.1) |
2 (8.3) |
Neuromuscular Disorders |
3 (6.1) |
1 (4.2) |
Paresthesia |
4 (8.2) |
1 (4.2) |
Respiratory System |
||
Respiratory Disorder |
4 (8.2) |
1 (4.2) |
Skin and Appendages |
||
Skin Reactions |
6 (12.2) |
0 (0) |
Urogenital System |
||
Urinary Disorders |
5 (10.2) |
4 (16.7) |
Considerations for Testosterone Measurement
- Based on assays available 40 years ago, surgical castrate levels of testosterone were reported as <50 mg/dL6,7
- US FDA and American Urological Association (AUA) currently define medical castration as testosterone levels <50 ng/dL8,9
- Current testosterone assay techniques are more rapid and accurate6
- Surgical castrate level using current assays determined as median 15 ng/dL (95% Cl: 12-17 ng/dL)6
- <20 ng/dL is the recommended goal for ADT therapy by the European Association of Urology Guidelines (EAU) 2023 and Bethesda consensus 20117,10
- No robust clinical data have clearly established the value of achieving testosterone <20 ng/dL6,10
ADT=androgen deprivation therapy; FDA=Food and Drug Administration.
Post hoc analysis of combined 4- and 6-month data, including pooled data
In a combined post hoc analysis of 2 phase 3 open-label studies in patients with histologically confirmed prostate adenocarcinoma, serum testosterone levels were pooled at each common time point to determine the number and proportion of patients who had levels ≤20 ng/dL. Assays with similar testosterone sensitivity were used.11
Limitations
Post hoc analyses are not powered or tested to demonstrate statistically significant differences in treatment effect. Pooled data limitation: patients with histologically confirmed adenocarcinoma were evaluated. Cancer staging and other patient characteristics (eg, age, prior treatment) may have been different between studies. The relationship between testosterone values and clinical outcomes has not been established.
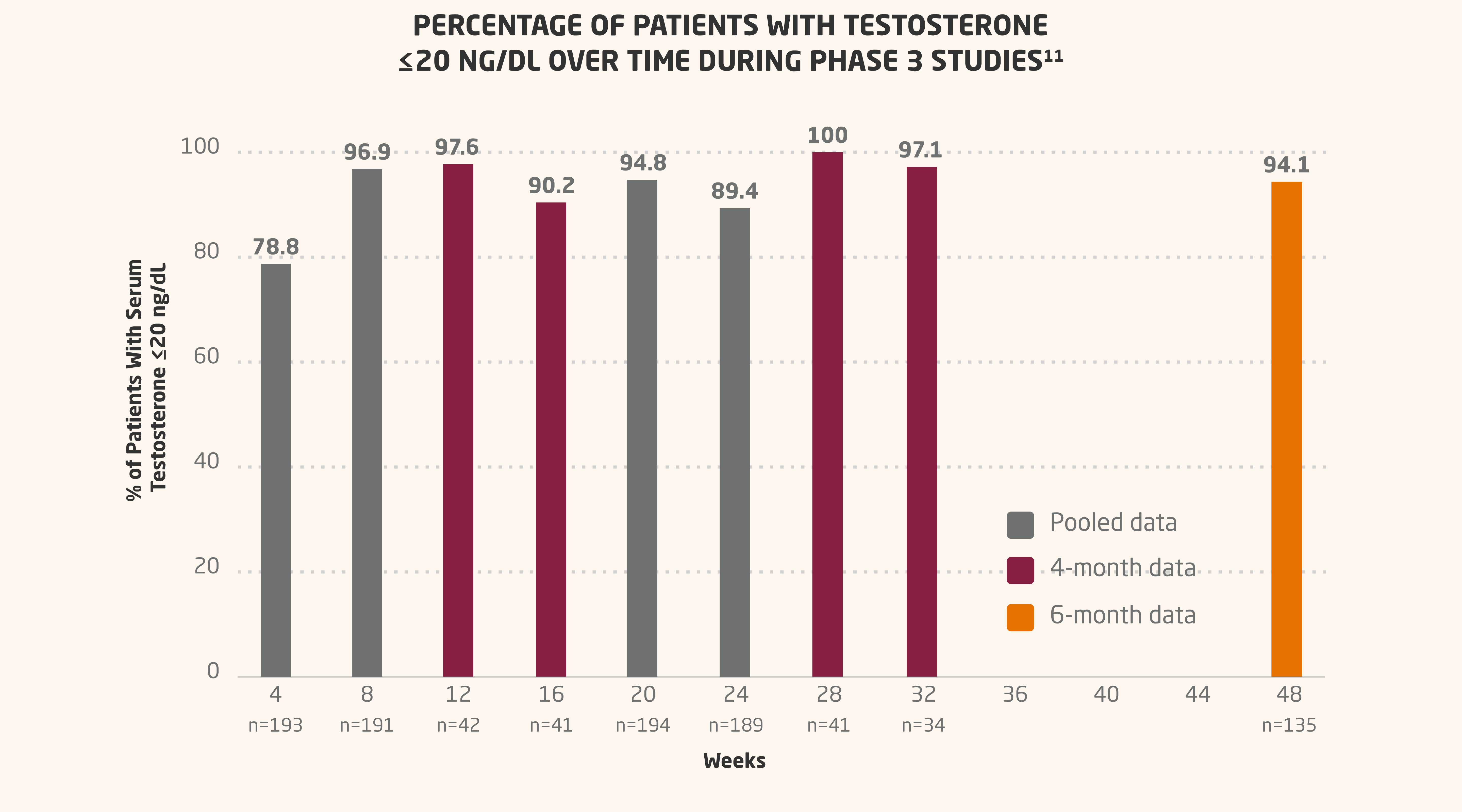
- In the pooled analysis, mean serum testosterone was suppressed to ≤20 ng/dL in 79% to 89% of patients from week 4 through week 2411
- In the 6-month formulation of LUPRON DEPOT, 94% of patients were suppressed ≤20 ng/dL at week 4811
In 2 nearly identical open-label, noncomparative, multicenter studies
Testosterone Suppression With 3-Month Dosing12
LUPRON DEPOT 22.5 MG: MEAN SERUM TESTOSTERONE CONCENTRATION THROUGH WEEK 241,12,13
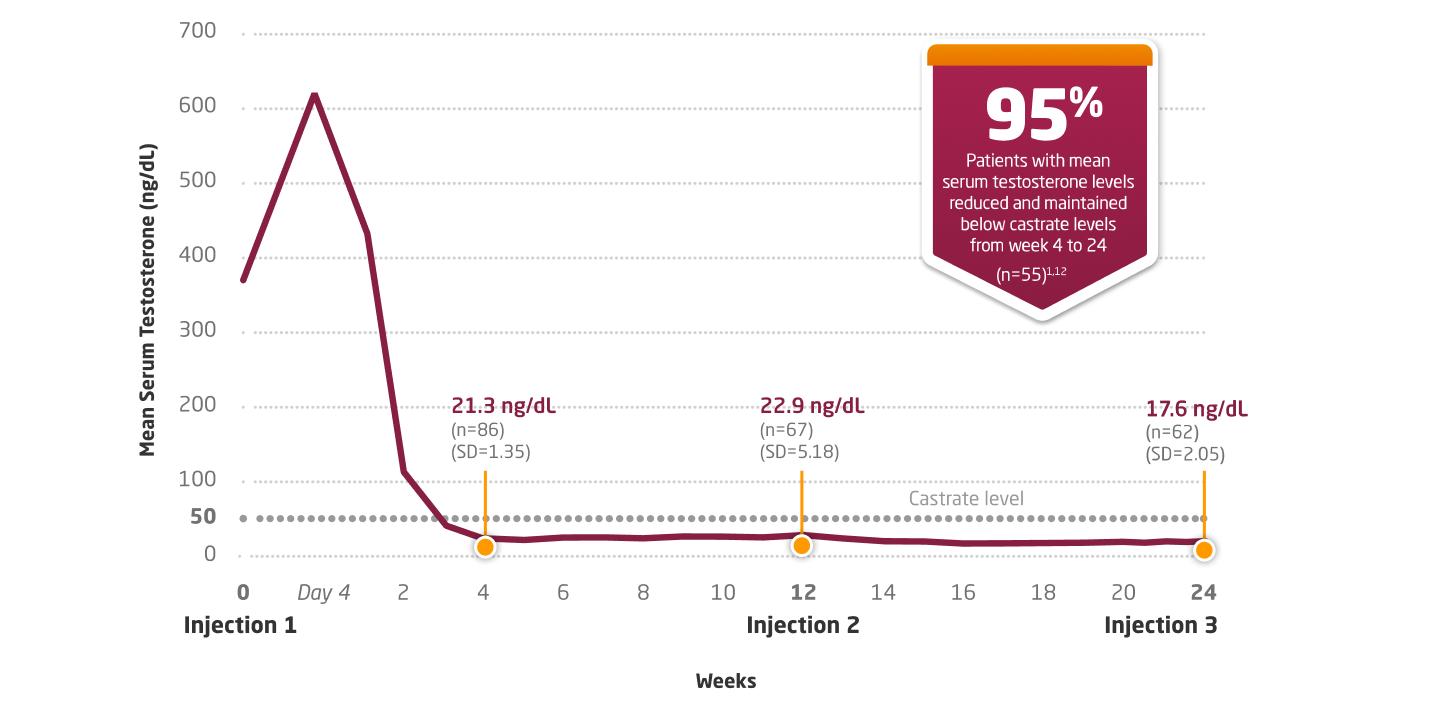
Primary Endpoint Results1,12
Castrate levels (≤50 ng/dL) of testosterone were achieved within 30 days of the initial injection in 95% (87/92) of patients in the 2 studies. After falling to castrate range, mean testosterone levels remained within the castrate range throughout each 12-week dosing interval.
Safety Data for 3-Month Dosing
| Adverse Events in ≥ 5% of Patients1 | N=94 | % |
|---|---|---|
| Body as a Whole | ||
Asthenia |
7 |
7.4 |
General Pain |
25 |
26.6 |
Headache |
6 |
6.4 |
Injection Site Reaction |
13 |
13.8 |
Cardiovascular System |
||
Hot Flashes/Sweats |
55 |
58.5 |
Digestive System |
||
GI Disorders |
15 |
16.0 |
Musculoskeletal System |
||
Joint Disorder |
11 |
11.7 |
Central/Peripheral Nervous System |
||
Dizziness/Vertigo |
6 |
6.4 |
Insomnia/Sleep Disorders |
8 |
8.5 |
Neuromuscular Disorders |
9 |
9.6 |
Respiratory System |
||
Respiratory Disorder |
6 |
6.4 |
Skin and Appendages |
||
Skin Reaction |
8 |
8.5 |
Urogenital System |
||
Testicular Atrophy |
19 |
20.2 |
Urinary Disorders |
14 |
14.9 |
Select a Dosing Schedule

"LUPRON DEPOT was the first [GnRHa] to develop and demonstrate efficacy in 1-month, 3-month, and 4-month doses, and later a 6-month dosing option, as well.”
GnRHa=gonadotropin-releasing hormone agonist.



